EMA’s Critical Medicines Regulation
Everything you need to know
EMA’s Critical Medicines Regulations in a nutshell
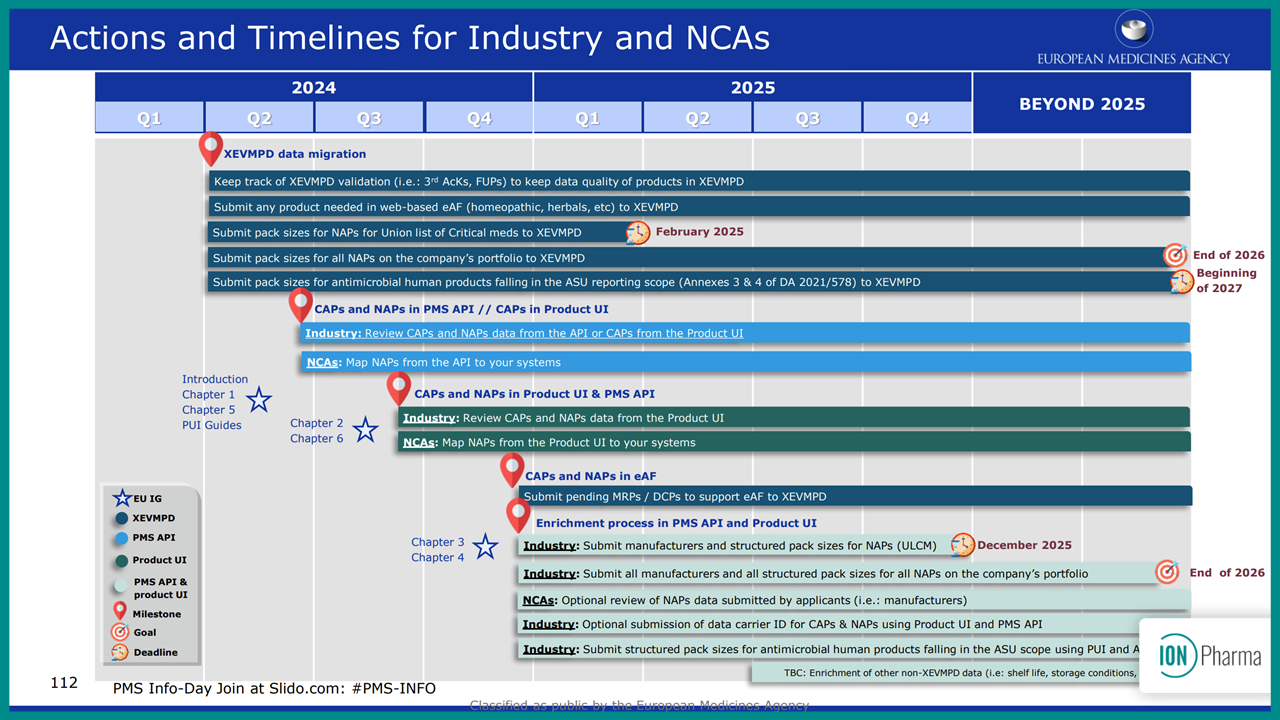
The European Medicines Agency (EMA) has implemented new Critical Medicines Regulations to address and manage medicine shortages in the EU. These regulations categorize medicines into three distinct groups which are tracked through the European Shortages Monitoring Platform (ESMP). This requires Marketing Authorization Holders (MAHs) to report supply data beginning in February 2025.
Article
Managing Medicine Shortages in the EU and the impact on Marketing Authorization Holders
The European Medicines Agency (EMA) has introduced regulations to improve the availability of critical medicines across the EU, categorizing them for different scenarios. Beginning February 2025, Marketing Authorization Holders will face new reporting requirements to support the European Shortages Monitoring Platform, ensuring timely access to essential medicines.
The time to act is now
Are you ready to navigate the new regulations? Do you understand their impact on your product data? Contact us today for expert guidance and support.