Ennov Quality Suite
Improve efficiency and compliance with a comprehensive QMS
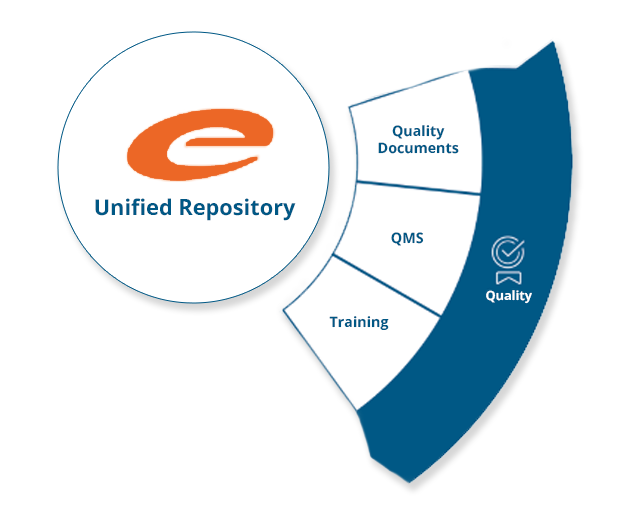
Innovating Quality Management
Ennov’s Quality suite is a quality management solution that not only improves operational efficiency by providing a true unified data repository, but also ensures compliance with industry standards such as 21 CFR Part 11, GxP and ISO.
The Quality Suite provides a predefined inventory of quality documentation, processes and workflows that are based on accepted industry standards and best demonstrated practices. This allows users of the Ennov Quality Suite to get their system into production quickly and start realizing their return on investment.
Differentiators

The Unified compliance platform shares a common user interface and data repository.

The application is fully configured and fully configurable.

The intuitive User Experience is unrivaled and is the key to high user adoption rates.

Dashboard data visualization that represent performance metrics in multiple ways.
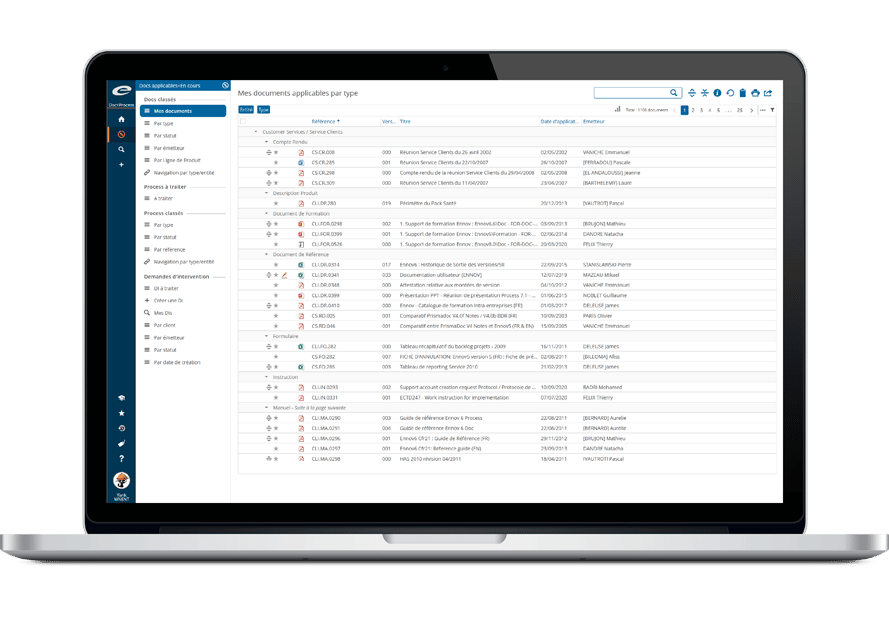
Doc for Quality
Manage Procedure, Manufacturing, Validation and other Quality Documentation with Ennov Doc.
Maintaining a paper-based, ISO-compliant document system is cumbersome, time-consuming and error-prone. Increase your productivity by automating repetitive tasks of managing quality documents. Maintain clear overview of the document lifecycle and proactively monitor workflow status to improve the quality system efficiency.
Collaborate, co-author, proofread, comment and sign documents electronically within the system including automatic email notifications and read receipts. Controlled printing, periodic reviews, automated document retention and archival schedules and full compliance with US FDA’s 21 CFR part 11 take the worry out of audits and quality inspections.
Process
Take control of Deviations, Non Conformances, CAPAs, Customer Complaints, Audits and more with Ennov Process.
Unreliable information, fragmented processes and inadequate monitoring can jeopardize your organization’s quality certification. Ennov Process offers a complete solution for managing all of your quality processes.
Streamline your processes critical to managing quality cases (e.g. CAPAs, deviations, non-conformances and customer complaints). A complete suite of to-do lists, smart email notifications, and reminders help you stay ahead of deadlines, increase productivity and take control of your quality program.

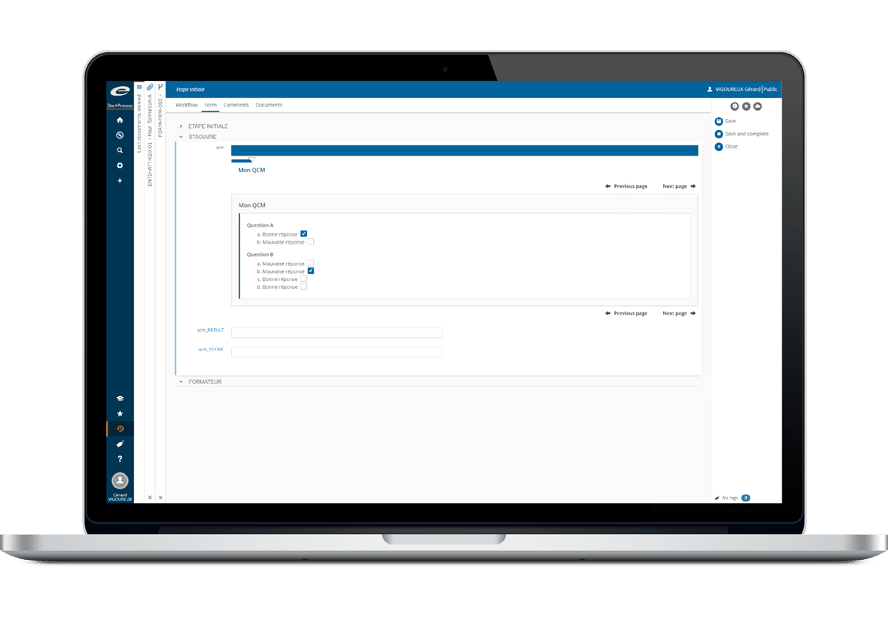
Training
Gain confidence that all employees have obtained the skills and knowledge required to effectively perform their work with Ennov Training.
Making sure your employees receive the appropriate training on internal policies and procedures is key to both individual and corporate performance. Ennov Training gives you confidence that all employees have obtained the requisite skills and knowledge required to effectively perform their work in compliance with corporate and industry quality standards.
Ennov Training is designed with both Training Administrators and Trainees in mind and streamlines the entire learning management process. Automated reports provide the visibility required to verify that each employee has been properly trained – greatly simplifying audits and quality inspections.
Discover Ennov’s Quality Suite
Get in touch to learn more