EMA Critical Medicines Regulations
Managing Medicine Shortages in the EU
27 September 2024 | Reading time: 3 minutes
Strengthening Medicine Availability in the EU
The European Medicines Agency (EMA) has introduced new regulations to tackle medicine shortages, following the disruptions caused by the COVID-19 pandemic. These regulations ensure the availability of critical medicines across the EU by organizing medicines into three categories:
1. Normal circumstances: Medicines on the Union list of critical medicines
2. Crisis preparedness: Medicines on the list to be monitored for MSSG-Led crisis preparedness
3. Crisis management: Medicines on the list of critical medicines for a public health emergency
The Union list of critical medicines (ULCM) prioritizes medicines vital to patient care and healthcare systems, to strengthen their supply chains and minimise the risk of supply disruptions. EMA also uses this list of critical medicines in the establishment of the Product Management System (PMS) to support the standardisation of product information in the EU and EEA.
Video
EMA’s Critical Medicines Regulations in a nutshell
European Shortages Monitoring Platform (ESMP)
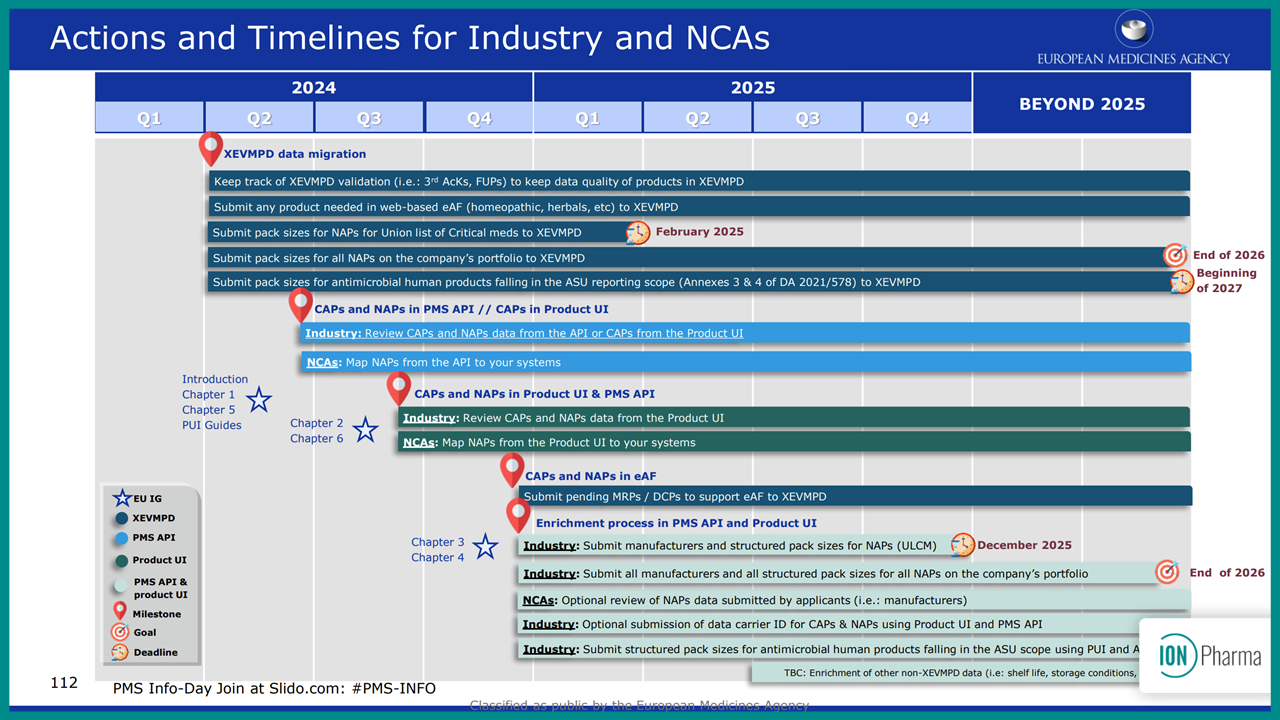
To support this effort, the European Shortages Monitoring Platform (ESMP) will monitor the availability of critical medicines. From February 2025, Marketing Authorization Holders (MAHs) must submit data on the supply of critical medicines, for both centrally authorized (CAPs) and nationally authorized products (NAPs). This can be done manually or through automated machine-to-machine interfaces. The transition period starts from November 2024 until end of 2026, with the first deadline set for submitting pack size information for those products listed on the ULCM before February 2025.
Impact on Marketing Authorization Holders (MAHs)
MAHs will experience different reporting requirements based on their product authorizations that are on the Union list of critical medicines. Centrally Authorized Products (CAPs) will initially see minimal change, as pack size and manufacturing data have already been submitted through existing systems. Nationally Authorized Products (NAPs), however, must submit pack size information to the PMS or xEVMPD before the February 2025 deadline. Manufacturing information needs to be submitted later that year.
In crisis situations (medicine categories 2 and 3), MAHs will have just 14 days to submit pack size information. These measures aim to streamline the EMA’s response to potential medicine shortages, ensuring timely availability of essential medicines across the EU.
For many companies this will mean a major impact on their regulatory data, system (RIMS), and resources against very short implementation timelines.
Navigating EMA’s new Critical Medicines Regulations can be challenging. We are here to help Marketing Authorization Holders (MAHs) meet deadlines and ensure compliance.
The time to act is now! Contact us today for expert guidance and support to keep your product data in line with these new regulations.