Electronic Quality
Management System
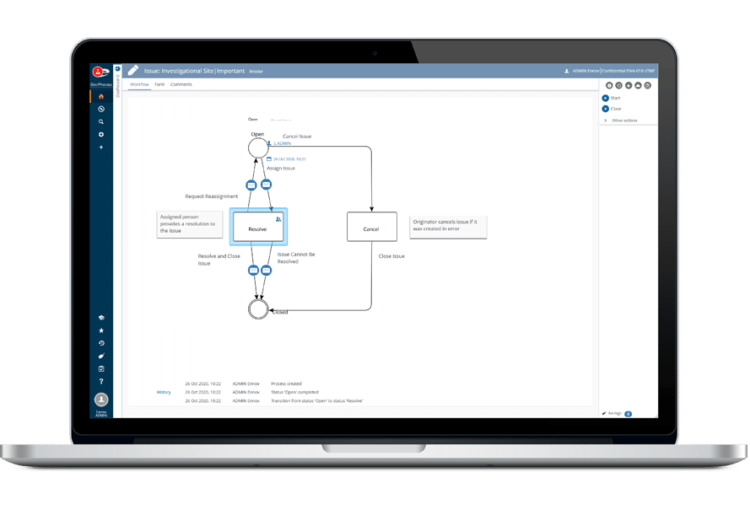
Management of Deviations, Customer Complaints and Audits with built-in Change Control
![]() Automate quality processes and streamline workflows
Automate quality processes and streamline workflows
![]() Access real-time data and analytics on quality metrics and performance indicators
Access real-time data and analytics on quality metrics and performance indicators
![]() Ensure compliance and adhere to regulations
Ensure compliance and adhere to regulations
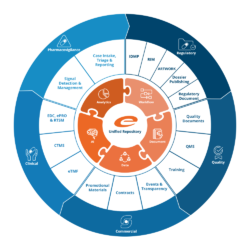
Easy Access to Key Quality Processes
Audits
Integrate audit readiness into your business.
Change Control
Manage changes effectively with complete traceability.
Complaints
Manage complaints to ensure compliance and customer satisfaction.
Deviations
Automate workflows for any deviation event.
End-to-end implementation support
As an implementation partner ION Pharma supports your project from start to finish. Starting with User Requirements Specifications to meet your specific needs all the way to system implementation and validation, and comprehensive training for trainers and end-users.
Implementation Types
- Out-of-the-Box – Standard Functionality to get you going
- ION Pharma’s Core Model – Industry Best Practice
- Turnkey – Client configured and ready to go

Chantal van Gorp
Chantal has over 20 years of experience in Quality Assurance in the pharmaceutical (Manufacturing and IT) industry. She has had roles in Production and Quality Assurance.
Leveraging a Unified Platform
Designed for Regulated Content, Quality and Information Management
We believe the only way in achieving the availability of correct, complete, and compliant information, for the users that need to have it when they need to have it starts with a single unified system at the core of your processes. Therefore we partner with Ennov who have translated that philosophy in a truly single unified platform.
Ready to see more?
Get ahead with an Electronic Quality Management System (eQMS)
Contact Us
We are here to help, whether you are looking for advice or immediate support. Let’s get in touch.